Hydrogen -1 Helium-4 Lithium = 7 Beryllium = 9 Boron = 11 Carbon = 12 Nitrogen = 14 Oxygen = 16 Fluorine = 19 Neon = 20 Sodium = 23 Magnesium = 24 Aluminum = 27 Silicon = 28 Phosphorus =31 Sulfur = 32 Chlorine =35 Argon = 40 Potassium = 39 Calcium. Hydrogen is estimated to make up more than 90% of all the atoms three quarters of the mass of the universe! This element is found in the stars, and plays an important part in powering the universe through both the proton-proton reaction and carbon-nitrogen cycle. Atomic Mass Isotopic Composition Standard Atomic Weight Notes: 1: H: 1: 1.007 825 032 23(9) 0.999 885(70) 1.007 84, 1.008 11 m: D: 2: 2.014 101 778 12(12) 0.000 115(70) T: 3: 3.016 049 2779(24).
Mass Numbers - How to find Mass Numbers
The mass number is established by rounding the atomic weight to the nearest whole number. The Periodic Table with Atomic Masswill give you the atomic weight, or atomic mass, of the elements. The chemical properties of an element are determined by its Atomic Number not its Mass Number which is why atomic numbers are shown on the Periodic table whilst Mass Numbers are not. Mass numbers equal the total number of heavy, or massive, particles in the nucleus. Subtracting the Atomic number from the Mass Number equals the number of neutrons in the nucleus.

Mass Numbers = Atomic Weight of Element, rounded to nearest whole number
Number of Neutrons = Mass Number - Atomic Number

Mass Numbers
Mass Numbers - The Mass Numbers of all of the elements
So, if we know the number of protons and neutrons in an atom we can determine the mass number. The unique chart below has been created by www.elementalmatter.info and details all of the elements in the Periodic table, the numbers of protons, the numbers of neutrons and the mass numbers of atoms which relate to the elements.
Mass numbers - Examples of Mass Numbers
The following examples provide details of how to calculate the mass number.
- Example 1 - mass number of Gold: The element Gold (Symbol Au) has the Atomic Number of 79. The number of protons in atom of gold is therefore 79. Gold has the Atomic Mass weight of 196.97. Round to the nearest whole number. The mass number of gold is therefore 197.
- Example 2 - mass number of Silver: The element Silver (Symbol Ag) has the Atomic Number of 47. The number of protons in atom of silver is therefore 47. Silver has the Atomic Mass weight of 107.87. Round to the nearest whole number. The mass number of silver is therefore 108.
- Example 3 - mass number of Neon: The element Neon (Symbol Ne) has the Atomic Number of 10. The number of protons in atom of neon is therefore 10. Neon has the Atomic Mass weight of 20.18. Round to the nearest whole number. The mass number of neon is therefore 20.
Mass Numbers = Atomic Weight of Element, rounded to nearest whole number
Mass numbers - Chart of Mass Numbers
The details all of the elements in the Periodic table, the numbers of protons, the numbers of neutrons and the mass numbers of atoms which relate to the elements in the Periodic Table.
Chart of Mass Numbers
| Atomic Number | Name of Element | Symbol of Element | Mass Numbers | Name of Element |
| 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 | Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon Cesium Barium Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon Francium Radium Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Ununbium Ununtrium Ununquadium Ununpentium Ununhexium Ununseptium Ununoctium | H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo | 1 4 7 9 11 12 14 16 19 20 23 24 27 28 31 32 35 40 40 40 45 48 51 52 55 56 58 58 64 65 70 73 75 79 80 84 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 133 137 139 140 141 144 145 150 152 157 159 163 165 167 169 173 175 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 223 226 227 232 231 238 237 244 243 247 247 251 252 257 258 259 262 261 268 263 264 269 268 272 273 277 286 289 288 292 292 293 | Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Sodium Magnesium Aluminium Silicon Phosphorus Sulfur Chlorine Argon Potassium Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium Germanium Arsenic Selenium Bromine Krypton Rubidium Strontium Yttrium Zirconium Niobium Molybdenum Technetium Ruthenium Rhodium Palladium Silver Cadmium Indium Tin Antimony Tellurium Iodine Xenon Cesium Barium Lanthanum Cerium Praseodymium Neodymium Promethium Samarium Europium Gadolinium Terbium Dysprosium Holmium Erbium Thulium Ytterbium Lutetium Hafnium Tantalum Tungsten Rhenium Osmium Iridium Platinum Gold Mercury Thallium Lead Bismuth Polonium Astatine Radon Francium Radium Actinium Thorium Protactinium Uranium Neptunium Plutonium Americium Curium Berkelium Californium Einsteinium Fermium Mendelevium Nobelium Lawrencium Rutherfordium Dubnium Seaborgium Bohrium Hassium Meitnerium Darmstadtium Roentgenium Ununbium Ununtrium Ununquadium Ununpentium Ununhexium Ununseptium Ununoctium |
| Atomic Number | Name of Element | Symbol of Element | Mass Numbers | Name of Element |
Chart of Mass Numbers
Atomic Mass
Atomic mass is based on a relative scale and the mass of 12C (carbon twelve) is defined as 12 amu.
Why do we specify 12C? We do not simply state the the mass of a C atom is 12 amu because elements exist as a variety of isotopes.
Carbon exists as two major isotopes, 12C, and 13C (14C exists and has a half life of 5730 y, 10C and 11C also exist; their half lives are 19.45 min and 20.3 days respectively). Each carbon atom has the same number of protons and electrons, 6. 12C has 6 neutrons, 13C has 7 neutrons, and 14C has 8 neutrons and so on. Since there are a variety of carbon isotopes we must specify which C atom defines the scale.
All the masses of the elements are determined relative to 12C.
By the way, the mass of an element is not equal to the sum of the masses of the subatomic particles of which the element is made!
Average Atomic Mass
Since many elements have a number of isotopes, and since chemists rarely work with one atom at a time, chemists use average atomic mass.
On the periodic table the mass of carbon is reported as 12.01 amu. This is the average atomic mass of carbon. No single carbon atom has a mass of 12.01 amu, but in a handful of C atoms the average mass of the carbon atoms is 12.01 amu.
Why 12.01 amu?
If a sample of carbon was placed in amass spectrometer the spectrometer would detect two different C atoms, 12C and 13C.
The natural abundances of 14C, 10C and 11C are so low that most mass spectrometers cannot detect the effect these isotopes have on the average mass. 14C dating is accomplished by measuring the radioactivity of a sample, not by actually counting the number of 14C atoms.
The average mass of a carbon is calculated from the information the mass spectrometer collects.
The mass spectrometer reports that there are two isotopes of carbon,
98.99% of the sample has a mass of 12 amu (not a surprise since this is the atom on which the scale is based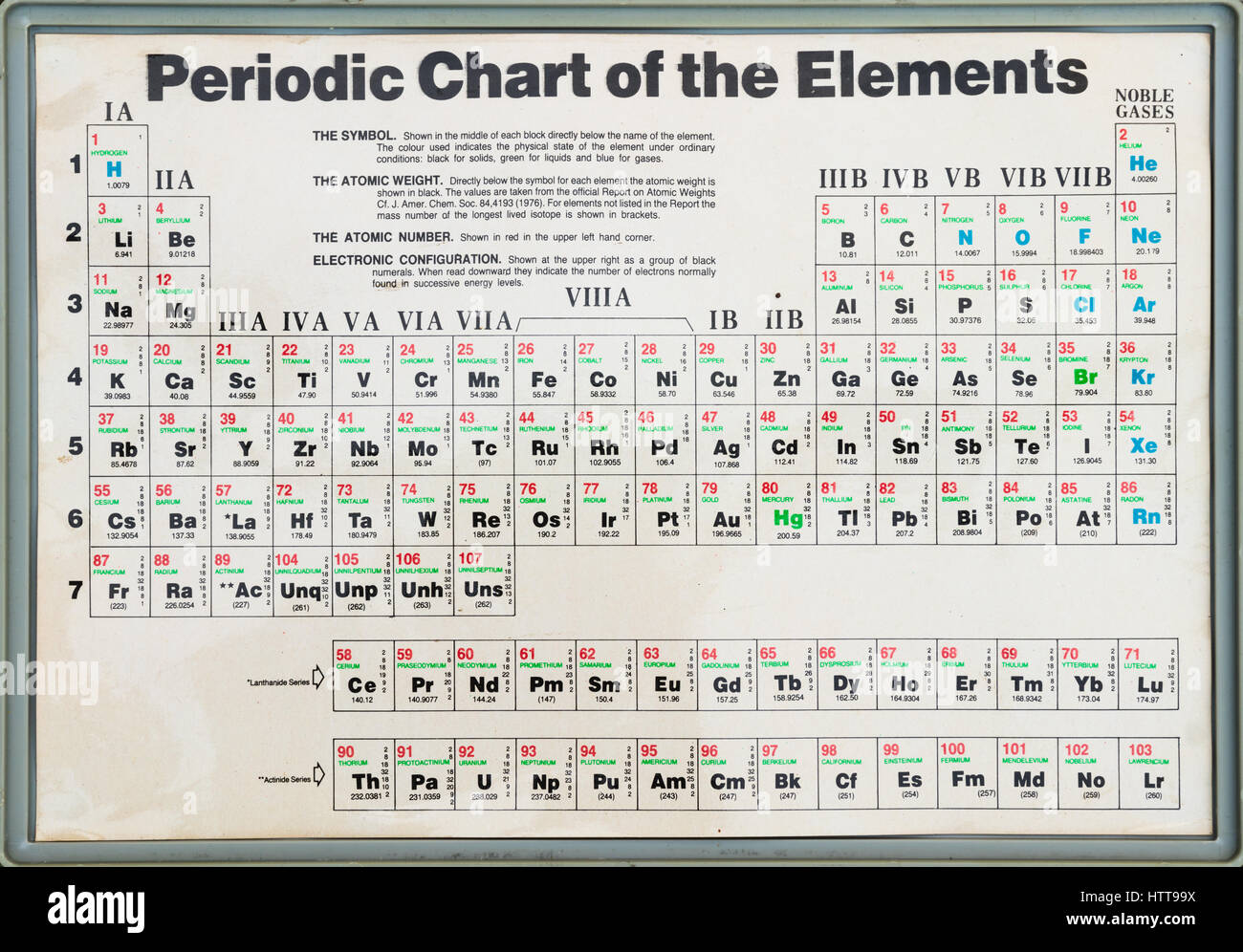 ).
). 1.11% of the sample has a mass of 13.003355 amu (this isotope is 1.0836129 times as massive as 12C)
The average mass is simply a weighted average.
ave. mass = 12.01 amu
Atomic Mass Of All Elements Chart
(Yes, the number 12.01 has the right number of significant figures, even though 1.11% only has 3 significant figures.)
If we know the natural abundance (the natural abundance of an isotope of an element is the percent of that isotope as it occurs in a sample on earth) of all the isotopes and the mass of all the isotopes we can find the average atomic mass. Nyan catwatermelon gaming. The average atomic mass is simply a weighted average of the masses of all the isotopes.
(Yes, the sig figs are correct.)
Another kind of question could be asked..
Copper has two isotopes 63Cu and 65Cu. The atomic mass of copper is 63.54. The atomic masses of 63Cu and 65Cu are 62.9296 and 64.9278 amu respectively; what is the natural abundance of each isotope?
substituting gives
One equation and two unknowns..is there another equation? If there is another equation we would have two equations and two unknowns, and a system of two equations and two unknowns is solvable.
Since there are only two major isotopes of Cu we know that
or
(eq. B)
Use eq. B to substitute for %63Cu in eq. A.
To the correct number of significant figures
Of course, a question like the one above could be turned around another way.
Gallium, atomic mass 69.72 amu, has two major isotopes, 69Ga, atomic mass 68.9257 amu, and 71Ga. If the natural abundance of each isotope is 60.00 and 40.00 % respectively what is the mass (in amu) of 71Ga.
Relative Atomic Mass List
The mole
What is the relative mass of 1 C atom as compared to 1 H atom?
What is the relative mass of 100 C atoms as compared to 100 H atoms?

What is the relative mass of 1 W atom as compared to 1 H atom?
What is the relative mass of 100 W atoms as compared to 100 H atoms?
The point here? As long as the number of atoms remains the same the relative mass does not change.

Atoms are small, and it is possible to place 1.0079 g of H on a balance (possible but not easy in the case of hydrogen).
It is also possible to place 183.9 g W, or 12.01 g of C on a balance.
Now, I state with absolute certainty that I have placed the same number of atoms on each balance! How do I know? I know because the relative masses of the samples on the balance, are the same as the relative masses of the individual atoms.
W:H = (183.9 g/1.0079 g):1 = 182:1C:H = (12.01 g/1.0079 g):1 = 11.92:1
The number of atoms I placed on the balance is know as a mole.
For many years the number of atoms in a mole remained unknown; however, now it is know that a mole of atoms contains 6.02214 x 1023 atoms.
So, the periodic table provides us with a great deal of information.
The periodic table lists
the mass of an atom in amu,the mass of a mole of atoms (i.e. the molar mass) in grams,
and the mass of 6.02214 x 1023 atoms in grams
The atomic mass of C is 12.01 amu. What is the mass of 1 C atom?