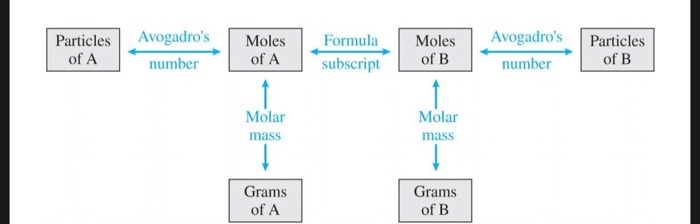
0.450 mole of Fe contains how many atoms? Solution: Start from the box labeled 'Moles of. National Mole Day site Avogadro's number NA = 6.02 × 10 23, like any pure number, is dimensionless. However, it also defines the mole, so we can also express NA as 6.02 × 1023 mol–1; in this form, it is properly known as Avogadro's constant. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms, molecules, or other specified particles. The number of units in a mole also bears the name Avogadro’s number, or Avogadro’s constant, in honor of the Italian physicist Amedeo Avogadro.
- Molecules Moles Avogadro's Number
- Mole Avogadro's Number
- Mole Avogadro's Number Worksheet
- Avogadro's Number And Mole
The number of moles in a system can be determined using the atomic mass of an element, which can be found on the periodic table. This mass is usually an average of the abundant forms of that element found on earth. An element's mass is listed as the average of all its isotopes on earth.
Avogadro's Constant
One mole of oxygen atoms contains (6.02214179 times 10^{23}) oxygen atoms. Also, one mole of nitrogen atoms contains (6.02214179 times 10^{23}) nitrogen atoms. The number (6.02214179 times 10^{23}) is called Avogadro's number ((N_A)) orAvogadro's constant, after the 19th century scientist Amedeo Avogadro.
Each carbon-12 atom weighs about (1.99265 times 10^{-23}; g); therefore,
[(1.99265 times 10^{-23}; g) times (6.02214179 times 10^{23}; atoms) = 12; g; text{ of carbon-12} nonumber ]
Applications of the Mole
The mass of a mole of substance is called the molar mass of that substance. The molar mass is used to convert grams of a substance to moles and is used often in chemistry. The molar mass of an element is found on the periodic table, and it is the element's atomic weight in grams/mole (g/mol). If the mass of a substance is known, the number of moles in the substance can be calculated. Converting the mass, in grams, of a substance to moles requires a conversion factor of (one mole of substance/molar mass of substance).
The mole concept is also applicable to the composition of chemical compounds. For instance, consider methane, CH4. This molecule and its molecular formula indicate that per mole of methane there is 1 mole of carbon and 4 moles of hydrogen. In this case, the mole is used as a common unit that can be applied to a ratio as shown below:
[2 text{ mol H } + 1 text{ mol O }= 1 text{ mol } ce{H2O} nonumber]
In this this chemical reactions, the moles of H and O describe the number of atoms of each element that react to form 1 mol of (ce{H_2O}).


To think about what a mole means, one should relate it to quantities such as dozen or pair. Just as a pair can mean two shoes, two books, two pencils, two people, or two of anything else, a mole means 6.02214179×1023 of anything. Using the following relation:

[text{1 mole} = 6.02214179 times 10^{23}]
is analogous to saying:
[text{1 Dozen} = text{12 eggs}]
It is quite difficult to visualize a mole of something because Avogadro's constant is extremely large. For instance, consider the size of one single grain of wheat. If all the people who have existed in Earth's history did nothing but count individual wheat grains for their entire lives, the total number of wheat grains counted would still be much less than Avogadro's constant; the number of wheat grains produced throughout history does not even approach Avogadro's Number.
Example (PageIndex{1}): Converting Mass to Moles
How many moles of potassium ((ce{K})) atoms are in 3.04 grams of pure potassium metal?
Solution
In this example, multiply the mass of (ce{K}) by the conversion factor (inverse molar mass of potassium):
[dfrac{1; mol; K}{39.10; grams ;K} nonumber ]
39.10 grams is the molar mass of one mole of (ce{K}); cancel out grams, leaving the moles of (ce{K}):
[3.04; cancel{g; K} left(dfrac{1; mol; K}{39.10; cancel{g; K}}right) = 0.0778; mol; K nonumber ]
Similarly, if the moles of a substance are known, the number grams in the substance can be determined. Converting moles of a substance to grams requires a conversion factor of molar mass of substance/one mole of substance. One simply needs to follow the same method but in the opposite direction.
Example (PageIndex{2}): Converting Moles to mass
How many grams are 10.78 moles of Calcium ((ce{Ca}))?
Solution
Multiply moles of Ca by the conversion factor (molar mass of calcium) 40.08 g Ca/ 1 mol Ca, which then allows the cancelation of moles, leaving grams of Ca.
[10.78 cancel{;mol; Ca} left(dfrac{40.08; g; Ca}{1; cancel{mol; Ca}}right) = 432.1; g; Ca nonumber ]
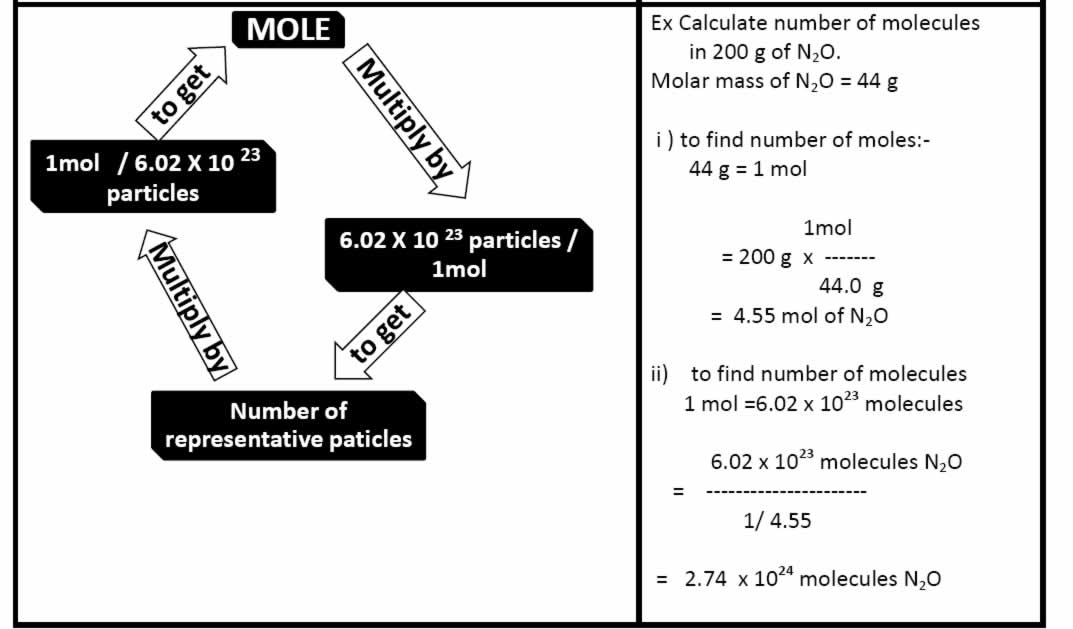
The total number of atoms in a substance can also be determined by using the relationship between grams, moles, and atoms. If given the mass of a substance and asked to find the number of atoms in the substance, one must first convert the mass of the substance, in grams, to moles, as in Example (PageIndex{1}). Then the number of moles of the substance must be converted to atoms. Converting moles of a substance to atoms requires a conversion factor of Avogadro's constant (6.02214179×1023) / one mole of substance. Verifying that the units cancel properly is a good way to make sure the correct method is used.
Example (PageIndex{3}): Atoms to Mass
How many atoms are in a 3.5 g sample of sodium (Na)?
Abouttn hindi. Solution
[3.5; cancel{g; Na} left(dfrac{1; mol; Na}{22.98; cancel{g; Na}}right) = 0.152; mol; Na nonumber ]
[0.152; cancel{mol; Na} left(dfrac{6.02214179times 10^{23}; atoms; Na}{1;cancel{ mol; Na}}right) = 9.15 times 10^{22}; atoms; of; Na nonumber ]
In this example, multiply the grams of Na by the conversion factor 1 mol Na/ 22.98 g Na, with 22.98g being the molar mass of one mole of Na, which then allows cancelation of grams, leaving moles of Na. Then, multiply the number of moles of Na by the conversion factor 6.02214179×1023 atoms Na/ 1 mol Na, with 6.02214179×1023 atoms being the number of atoms in one mole of Na (Avogadro's constant), which then allows the cancelation of moles, leaving the number of atoms of Na.
Using Avogadro's constant, it is also easy to calculate the number of atoms or molecules present in a substance (Table (PageIndex{1})). By multiplying the number of moles by Avogadro's constant, the mol units cancel out, leaving the number of atoms. The following table provides a reference for the ways in which these various quantities can be manipulated:
| Known Information | Multiply By | Result |
|---|---|---|
| Mass of substance (g) | 1/ Molar mass (mol/g) | Moles of substance |
| Moles of substance (mol) | Avogadro's constant (atoms/mol) | Atoms (or molecules) |
| Mass of substance (g) | 1/Molar mass (mol/g) × Avogadro's constant (atoms/mol)) | Atoms (or molecules) |
Example (PageIndex{4}): Mass to Moles
How many moles are in 3.00 grams of potassium (K)?
Solution
[3.00 ; cancel{g; K} left(dfrac{1; mol; K}{39.10; cancel{g; K}}right) = 0.0767; mol; K nonumber ]
In this example, multiply the mass of K by the conversion factor:
[dfrac{1; mol; K}{39.10; grams; K} nonumber ]
39.10 grams is the molar mass of one mole of K. Grams can be canceled, leaving the moles of K.
Example (PageIndex{5}): Moles to Mass
How many grams is in 10.00 moles of calcium (Ca)?
Solution
This is the calculation in Example (PageIndex{2}) performed in reverse. Multiply moles of Ca by the conversion factor 40.08 g Ca/ 1 mol Ca, with 40.08 g being the molar mass of one mole of Ca. The moles cancel, leaving grams of Ca:
[10.00; cancel{mol; Ca} left(dfrac{40.08; g; Ca}{1;cancel{ mol; Ca}}right) = 400.8; grams ;of ;Ca nonumber ]
The number of atoms can also be calculated using Avogadro's Constant (6.02214179×1023) / one mole of substance.
Example (PageIndex{6}): Mass to Atoms
How many atoms are in a 3.0 g sample of sodium (Na)?
Solution
Convert grams to moles
[3.0; cancel{g; Na} left(dfrac{1; mol; Na}{22.98; cancel{g; Na}}right) = 0.130; mol; Na nonumber ]
Convert moles to atoms
[0.130548; cancel{ mol; Na} left(dfrac{6.02214179 times 10^{23}; atoms ;Na}{1; cancel{ mol; Na}}right) = 7.8 times 10^{22} ; atoms; of; ; Na nonumber ]
Summary
The mole, abbreviated mol, is an SI unit which measures the number of particles in a specific substance. One mole is equal to (6.02214179 times 10^{23}) atoms, or other elementary units such as molecules.
Problems
- Using a periodic table, give the molar mass of the following:
- H
- Se
- Ne
- Cs
- Fe
- Convert to moles and find the total number of atoms.
- 5.06 grams of oxygen
- 2.14 grams of K
- 0.134 kg of Li
- Convert the following to grams
- 4.5 mols of C
- 7.1 mols of Al
- 2.2 mols of Mg
- How many moles are in the product of the reaction
- 6 mol H + 3 mol O → ? mol H2O
- 1 mol Cl + 1 mol Cl → ? mol Cl2
- 5 mol Na + 4 mol Cl → ? mol NaCl
Answers
- Question 2
- 1.008 g/mol
- 78.96 g/mol
- 20.18 g/mol
- 132.91g/mol
- 55.85 g/mol
- Question 2
2. 5.06g O (1mol/16.00g)= 0.316 mol of O
0.316 mols (6.022x1023 atoms/ 1mol) = 1.904x1023 atoms of O
3. 2.14g K (1mol/39.10g)= 0.055 mol of K
Molecules Moles Avogadro's Number
0.055 mols (6.022x1023 atoms/ 1mol) = 3.312x1022 atoms of K
4. 0.134kg Li (1000g/1kg)= 134g Li (1mol/6.941g)= 19.3 mols Li
19.3 (6.022x1023 atoms/ 1mol) = 1.16x1025 atoms of Li
- Question 3
- 4.5 mols of C (12.011g/1mol) = 54.05 g of C
- 7.1 mols of Al (26.98g/1mol) = 191.56 g of Al
- 2.2 mols of Mg (24.31g/1mol) = 53.48 g of MG
- Question 4
- 8. 6 mol H + 3 mol O → 3 mol H2O
- 9. 1 mol Cl + 1 mol Cl → 1 mol Cl2
- 10. 5 mol Na + 4 mol Cl → 4 mol NaCl + 1 mol Na (excess)
References
Mole Avogadro's Number
- Keenan, Charles W. and Wood, Jesse H. . General College Chemistry. 4th ed. New York: Haper and Row, 1971.
- Mortimer, Charles E. Chemistry a Conceptual Approach. 2nd ed. New York: Van Nostrand Reinhold, 1971.
- Jones, Loretta and Atkins, Peter. Chemistry: Molecules, Matter, and Change. 4th ed. New York: W.H. Freeman, 2000.
- Petrucci, Ralph H., Herring, Goeffrey F., Madura, Jeffrey D., and Bissonnette, Carey. General Chemistry: Principles and Modern Applications. 10th ed. New Jersey: Pearson Canada, 2011.
Mole Avogadro's Number Worksheet
Contributors and Attributions
Avogadro's Number And Mole
- Ryan Benoit (UCD), Michael Thai (UCD), Charlie Wang (UCD), Jacob Gomez (UCD)
